Alzheimer’s Therapeutics Market on Upward Trajectory Across the 7MM During the Forecast Period (2025–2034) Fueled by Drug Development Advances | DelveInsight
The Alzheimer’s disease market is anticipated to surge owing to the expected launch of emerging therapies, such as BioVie's Bezisterim (NE3107), AB Science's Masitinib (AB1010), Annovis Bio’s Buntanetap, Cassava Sciences' Simufilam (PTI-125), TauRx Therapeutics' Hydromethylthionine mesylate (TRx0237), Novo Nordisk's semaglutide (NN6535), and Eli Lilly's Remternetug (LY3372993), among others. Other therapies in the early stages of the trial are also being developed.
New York, USA, July 08, 2025 (GLOBE NEWSWIRE) -- Alzheimer’s Therapeutics Market on Upward Trajectory Across the 7MM During the Forecast Period (2025–2034) Fueled by Drug Development Advances | DelveInsight
The Alzheimer’s disease market is anticipated to surge owing to the expected launch of emerging therapies, such as BioVie's Bezisterim (NE3107), AB Science's Masitinib (AB1010), Annovis Bio’s Buntanetap, Cassava Sciences' Simufilam (PTI-125), TauRx Therapeutics' Hydromethylthionine mesylate (TRx0237), Novo Nordisk's semaglutide (NN6535), and Eli Lilly's Remternetug (LY3372993), among others. Other therapies in the early stages of the trial are also being developed.
DelveInsight’s Alzheimer's Disease Market Insights report includes a comprehensive understanding of current treatment practices, Alzheimer's disease emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan].
Key Takeaways from the Alzheimer's Disease Market Report
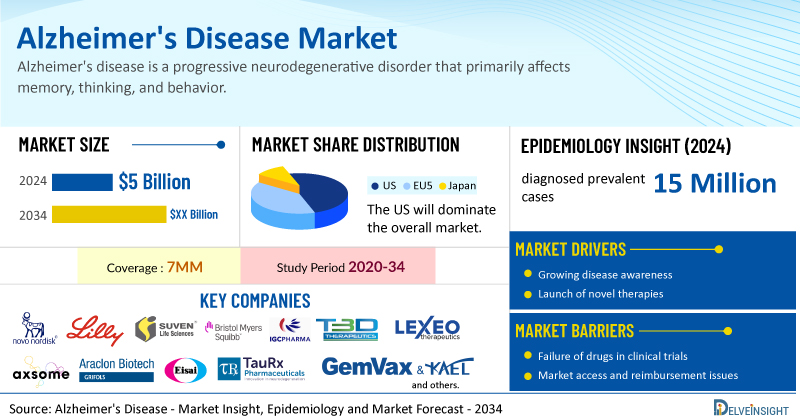
- According to DelveInsight’s analysis, the Alzheimer's Disease market size was found to be USD 5 billion in the 7MM in 2024.
- The United States accounted for the highest Alzheimer's Disease drug market size, approximately 50% of the total market size in 7MM in 2024, in comparison to the other major markets, i.e., EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- DelveInsight’s analysis indicates that in 2024, there were around 15 million diagnosed prevalent cases of Alzheimer's disease and nearly 100 million prevalent cases of preclinical Alzheimer's disease across the 7MM. These numbers are expected to grow steadily throughout the forecast period from 2025 to 2034, reflecting the rising aging population and improved diagnostic capabilities.
- According to DelveInsight’s estimates, the US had 5.5 million diagnosed prevalent cases of agitation associated with Alzheimer’s disease in 2024.
- Leading Alzheimer's disease companies developing emerging therapies, such as Novo Nordisk, Eli Lilly, Suven Life Sciences, Bristol Myers Squibb, IGC Pharma, T3D Therapeutics, Lexeo Therapeutics, Axsome Therapeutics, Araclon Biotech S.L., Eisai, TauRx Therapeutics, GemVax & KAEL, AC Immune SA, Johnson & Johnson, Longeveron, Vaccinex, Sinaptica Therapeutics, and others, are developing new Alzheimer's disease treatment drugs that can be available in the Alzheimer's disease market in the coming years.
- The promising Alzheimer's disease therapies in the pipeline include Masitinib (AB1010), Valiltramiprosate (ALZ-801), Mirodenafil (AR1001), Levetiracetam (AGB101), Blarcamesine (ANAVEX2-73), Buntanetap (ANVS401 or posiphen), Tricaprilin (CER0001), Bezisterim (NE3107), Semaglutide (NN6535), Remternetug (LY3372993), Masupirdine (SUVN-502), COBENFY (KarXT), IGC-AD1, T3D-959, LX 1001, AXS-05 (Bupropion/Dextromethorphan), ABvac40, E2814, Hydromethylthionine Mesylate (HMTM)/TRx0237, GV1001, ACI-35.030/JNJ-2056, LOMECEL-B (laromestrocel), Pepinemab, SinaptiStim System, and others.
Discover drug trials for Alzheimer's disease @ Alzheimer's Disease Treatments
Alzheimer's Disease Market Dynamics
The Alzheimer's disease market dynamics are expected to change in the coming years. Recent advances in structural and functional MRI techniques, along with biomarker assessments, have enhanced the understanding of anatomical and physiological brain changes in Alzheimer’s disease, supporting a robust drug development pipeline that includes a range of molecules targeting amyloid, tau abnormalities, inflammation, and synaptic dysfunction; this creates a window of opportunity for companies to leverage these insights to evaluate novel, safe, and effective therapies with convenient dosing and lower costs to improve treatment compliance and adherence.
As potential therapies are being investigated for the treatment of Alzheimer's disease, it is safe to predict that the treatment space will significantly impact the Alzheimer's disease market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate are expected to drive the growth of the Alzheimer's disease market in the 7MM.
However, several factors may impede the growth of the Alzheimer's disease market. Drug development for the disease has been hindered by a misunderstanding of its mechanisms, inconsistent protocols relying on single-target approaches, and poor project management, all contributing to high failure rates, prolonged timelines, and a low conversion to marketed products; further complicating progress are the disease’s variable progression, limited and costly biomarker-based diagnostic tools, especially in later stages, and growing challenges faced by traditional drug classes like acetylcholinesterase inhibitors and NMDA receptor antagonists due to generic erosion.
Moreover, Alzheimer's disease treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the Alzheimer's disease market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the Alzheimer's disease market growth.
Alzheimer's Disease Treatment Market
Alzheimer's disease currently has no known cure. However, early diagnosis and consistent treatment play a crucial role in alleviating clinical symptoms. Detecting the disease in its initial stages allows for timely symptomatic treatment, better management of behavioral symptoms, and the adoption of lifestyle interventions that may help reduce the risk of dementia and slow the progression of the disease.
Most existing dementia treatments aim to manage symptoms by modulating neurotransmitter activity in the brain. These therapies typically enhance the function of neurotransmitters like acetylcholine, serotonin, and noradrenaline, or inhibit the activity of others such as glutamate and dopamine. Given the potential for adverse effects, treatment plans must be tailored to each patient, taking into account existing health conditions and possible drug interactions, particularly those affecting heart function and drug metabolism. For over a decade, medications such as acetylcholinesterase inhibitors (AChEIs) and memantine, an NMDA receptor antagonist, have been available in the U.S. for symptomatic relief.
In recent years, significant progress in Alzheimer's research has led to a better understanding of disease mechanisms and the identification of new therapeutic targets. In July 2023, the U.S. FDA approved LEQEMBI (lecanemab), developed by Biogen and Eisai, for the treatment of early-stage Alzheimer’s, including mild cognitive impairment and mild dementia. The drug was subsequently approved in Japan in September 2023 and received marketing authorization in the UK in August 2024, highlighting the diverse regulatory landscape across regions. Another breakthrough came with Eli Lilly’s KISUNLA (donanemab), which received U.S. FDA approval in July 2024 and is considered a major advancement in treating early-stage Alzheimer’s disease.
To know more about FDA-approved Alzheimer's disease drugs 2025, visit @ Alzheimer's Disease Treatment Research
Alzheimer's Disease Pipeline Therapies and Key Companies
The Alzheimer’s disease pipeline is burgeoning, with a range of global companies advancing therapies for this indication. Prominent players in the market include AB Science (Masitinib), BioVie (Bezisterim), Annovis Bio (Buntanetap), TauRx Therapeutics (Hydromethylthionine mesylate), Cassava Sciences (Simufilam), Eli Lilly and Company (Remternetug), Novo Nordisk (Semaglutide), Eisai (E2814), UCB Pharma (Bepranemab), and GemVax & KAEL (GV1001), among others.
BioVie’s NE3107 is an orally administered, small molecule that can cross the blood-brain barrier. It acts as an anti-inflammatory and insulin sensitizer by binding to extracellular signal-regulated kinase (ERK) and selectively suppressing inflammation. NE3107 blocks ERK/NFκB activation and reduces TNF production triggered by inflammatory agents like lipopolysaccharide. BioVie is also developing NE3107 for Parkinson’s disease, multiple myeloma, and prostate cancer.
Recently, the company shared Phase III trial results, where the primary efficacy endpoint did not achieve statistical significance due to patient exclusions. However, the trial’s adaptive design enables continued enrollment to potentially reach statistical significance for regulatory approval. Given the efficacy signals observed, BioVie plans to collaborate with the FDA and aims for a global launch by 2026.
AB Science’s Masitinib (AB1010) is an oral tyrosine kinase inhibitor that targets activated mast cells and microglia, neuroimmune cells implicated in Alzheimer’s disease, which can accumulate in the CNS at therapeutic levels. Evidence from a Phase II trial suggests masitinib may benefit patients with mild-to-moderate Alzheimer’s disease. A confirmatory Phase III study is currently in progress.
Buntanetap (formerly ANVS401 or posiphen) is a synthetically derived, orally bioavailable small molecule that functions as a Translational Inhibitor of Neurotoxic Aggregating Proteins (TINAPs). This unique mechanism offers a novel therapeutic approach for neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and Alzheimer’s associated with Down syndrome.
In February 2025, the company announced the initiation of its pivotal Phase III trial for early-stage Alzheimer’s. Following a successful End-of-Phase II meeting with the FDA in October 2024, Annovis Bio received clearance to move forward with Phase III trials. The FDA and the company have agreed on a development path that supports the submission of two NDAs: one for short-term and another for long-term efficacy.
Novo Nordisk’s Semaglutide (NN6535) is a once-daily GLP-1 receptor agonist being studied for Alzheimer’s disease. GLP-1 is a known neurotransmitter, and its receptors are found in various brain regions, including the striatum, hippocampus, and nucleus accumbens.
Semaglutide is thought to slow disease progression by reducing neuroinflammation and improving cellular, neural, and vascular function, potentially enhancing cognition and daily function through diverse biological pathways. The drug is currently under evaluation in two Phase III clinical trials, EVOKE and EVOKE+, in patients with early Alzheimer’s, both expected to complete in 2025.
The anticipated launch of these emerging therapies are poised to transform the Alzheimer's disease market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the Alzheimer's disease market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Discover more about Alzheimer's disease drug development pipeline @ Alzheimer's Disease Drug Development Pipeline 2025
Recent Developments in the Alzheimer's Disease Market
- In June 2025, INmune Bio Inc. reported results from its Phase 2 MINDFuL trial (NCT05318976), which assessed XPro™, a targeted soluble TNF inhibitor, in patients with early-stage Alzheimer's disease showing signs of inflammation through biomarkers.
- In June 2025, Eli Lilly and Company announced that the FDA had approved a label update for Amyvid (florbetapir F 18 injection), which is administered intravenously. Amyvid is utilized in brain imaging to assess the density of amyloid plaques in individuals with cognitive impairment being evaluated for Alzheimer’s disease or other causes of cognitive decline.
- In June 2025, InMed Pharmaceuticals Inc. reported new preclinical findings showing that INM-901 significantly reduces inflammation in ex vivo neuroinflammation models, reinforcing its promise as a potential treatment for Alzheimer’s disease.
- In April 2025, Biogen Inc. announced that the FDA granted Fast Track designation to BIIB080, an antisense oligonucleotide therapy targeting tau, for Alzheimer’s disease treatment, aiming to speed up its development and review.
- In February 2025, Annovis Bio reported the enrollment of the first patients in its pivotal Phase III clinical trial evaluating buntanetap for the treatment of early-stage Alzheimer’s disease.
- In February 2025, NKGen Biotech, Inc. received Fast Track designation from the FDA for its investigational drug, troculeucel, aimed at treating moderate Alzheimer's disease (AD).
Alzheimer's Disease Overview
Alzheimer's disease is a progressive neurodegenerative disorder that primarily affects memory, thinking, and behavior. It is the most common cause of dementia, especially in older adults, and is characterized by the gradual loss of neurons and the connections between them in the brain.
The disease typically begins with mild memory loss but advances over time to severe cognitive impairment, disorientation, mood changes, and difficulties in performing everyday tasks. As the condition progresses, patients often lose the ability to carry out even basic bodily functions, eventually leading to death.
The exact causes of Alzheimer's are not fully understood, but it is believed to result from a combination of genetic, environmental, and lifestyle factors. Key pathological features include the accumulation of beta-amyloid plaques and neurofibrillary tangles in the brain.
Alzheimer’s disease symptoms often start with forgetfulness, confusion, trouble with language or problem-solving, and mood or personality changes. Diagnosis involves a comprehensive assessment that includes medical history, cognitive testing, neurological exams, and imaging techniques such as MRI or PET scans. In some cases, biomarker tests using cerebrospinal fluid or blood may support the diagnosis, although these are not yet widely used in routine clinical practice.
Alzheimer's Disease Epidemiology Segmentation
The Alzheimer's disease epidemiology section provides insights into the historical and current Alzheimer's disease patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The Alzheimer's disease market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM, segmented into:
- Diagnosed Prevalent Cases of Alzheimer’s Disease
- Prevalent Cases of Preclinical Alzheimer’s Disease
- Age-specific Diagnosed Prevalent Cases of Alzheimer’s Disease
- Gender-specific Diagnosed Prevalent Cases of Alzheimer’s Disease
- Severity-specific Diagnosed Prevalent Cases of Alzheimer’s Disease
- Genotype-specific Diagnosed Prevalent Cases of Alzheimer’s Disease
- Diagnosed Prevalent Cases of Agitation in Alzheimer’s Disease
- Diagnosed Prevalent Cases of Psychosis in Alzheimer’s Disease
| Alzheimer's Disease Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Coverage | 7MM [The United States, the EU4 (Germany, France, Italy, and Spain) and The United Kingdom, and Japan]. |
| Alzheimer's Disease Market Size in 2024 | USD 5 Billion |
| Key Alzheimer's Disease Companies | Novo Nordisk, Eli Lilly, Suven Life Sciences, Bristol Myers Squibb, IGC Pharma, T3D Therapeutics, Lexeo Therapeutics, Axsome Therapeutics, Araclon Biotech S.L., Eisai, TauRx Therapeutics, GemVax & KAEL, AC Immune SA, Johnson & Johnson, Longeveron, Vaccinex, Sinaptica Therapeutics, Alpha Cognition, Biogen, Otsuka Pharmaceutical, Lundbeck, and others |
| Key Alzheimer's Disease Therapies | Masitinib (AB1010), Valiltramiprosate (ALZ-801), Mirodenafil (AR1001), Levetiracetam (AGB101), Blarcamesine (ANAVEX2-73), Buntanetap (ANVS401 or posiphen), Tricaprilin (CER0001), Bezisterim (NE3107), Semaglutide (NN6535), Remternetug (LY3372993), Masupirdine (SUVN-502), COBENFY (KarXT), IGC-AD1, T3D-959, LX 1001, AXS-05 (Bupropion/Dextromethorphan), ABvac40, E2814, Hydromethylthionine Mesylate (HMTM)/TRx0237, GV1001, ACI-35.030/JNJ-2056, LOMECEL-B (laromestrocel), Pepinemab, SinaptiStim System, ZUNVEYL, LEQEMBI, KISUNLA, REXULTI, and others |
Scope of the Alzheimer's Disease Market Report
- Therapeutic Assessment: Alzheimer's Disease current marketed and emerging therapies
- Alzheimer's Disease Market Dynamics: Key Market Forecast Assumptions of Emerging Alzheimer's Disease Drugs and Market Outlook
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Alzheimer's Disease Market Access and Reimbursement
Download the report to understand Alzheimer's disease clinical trials and drug development @ Alzheimer's Disease Diagnostics and Therapeutics Market
Table of Contents
| 1. | Alzheimer's Disease Market Key Insights |
| 2. | Alzheimer's Disease Market Report Introduction |
| 3. | Alzheimer's Disease Market Overview at a Glance |
| 4. | Alzheimer's Disease Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Alzheimer's Disease Treatment and Management |
| 7. | Alzheimer's Disease Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Alzheimer's Disease Marketed Drugs |
| 10. | Alzheimer's Disease Emerging Drugs |
| 11. | Seven Major Alzheimer's Disease Market Analysis |
| 12. | Alzheimer's Disease Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Alzheimer's Disease Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Alzheimer's Disease companies, including Biogen, AZTherapies, Cerecin, Neurotrope, Synaptogenix, INmune Bio, Cassava Sciences, EIP Pharma, Neuraly, AB Science, Cortexyme, Anavex Life Sciences, Athira Pharma, Time Therapeutics, Denali Therapeutics Inc., Alector Inc., Lexeo Therapeutics, TrueBinding, Inc., Vaccinex Inc., Annovis Bio Inc., Eisai Inc., Hoffmann-La Roche, Ionis Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Cognition Therapeutics, Merck Sharp & Dohme LLC, ImmunoBrain Checkpoint, AbbVie, AriBio Co., Ltd., Oryzon Genomics S.A., Eli Lilly and Company, Neurokine Therapeutics, Excelsior, Seelos Therapeutics, Inc., Janssen Research & Development, LLC, Shanghai Hengrui Pharmaceutical Co., Ltd., reMYND, Alzinova AB, VTBIO Co. LTD, BioVie Inc., Prothena Corporation plc, Coya Therapeutics, Inc., among others.
Agitation in Alzheimer’s Disease Market
Agitation in Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key agitation in Alzheimer’s disease companies, including Eli Lilly and Co, BioVie Inc., AB Science SA, Annovis Bio Inc., Cognition Therapeutics Inc., Coya Therapeutics Inc., Actinogen Medical Limited, AC Immune SA, Biogen Inc., Longeveron Inc., among others.
Psychosis in Parkinson’s and Alzheimer’s Disease Market
Psychosis in Parkinson’s and Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key psychosis in Parkinson’s and Alzheimer’s disease companies, including Sunovion Pharmaceuticals, Karuna Therapeutics, Vanda Pharmaceuticals, Suven Life Sciences, Enterin, Intra-Cellular Therapies, Merck Sharp & Dohme, among others.
Parkinson’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Parkinson’s disease companies, including UCB Biopharma SRL, Novartis, Annovis Bio, Supernus Pharmaceuticals, Inc., Britannia Pharmaceutical, Pharma Two B, Mitsubishi Tanabe Pharma (NeuroDerm), AbbVie, Cerevel Therapeutics, LLC, among others.
Alzheimer’s Disease Diagnostic Market
Alzheimer’s Disease Diagnostic Market Insights, Competitive Landscape and Market Forecast – 2032 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key Alzheimer’s disease diagnostic companies, including F. Hoffmann-La Roche Ltd., General Electric Company, 23andMe, Inc., Lilly, Fujirebio, Siemens Medical Solutions USA, Inc., Diadem srl., Todos Medical, DISCERN™, FUJIFILM Holdings America Corporation, Koninklijke Philips N.V., CANON MEDICAL SYSTEMS EUROPE B.V., Shimzadu Corporation., Laboratory Corporation of America® Holdings, Bruker, Magnetica., IMRIS, Deerfield Imaging, Inc., MR Solutions, Hyperfine, Inc., Neusoft Corporation, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
